Development of generics: From R&D to GMP In-house Training
The interface between Development and GMP units is one of the most exciting in the pharmaceutical industry. Mutual understanding of the different regulatory environment and the functional priorities is a key success factor. Conflicts, mostly resulting from different approaches and priorities, can have negative impact on business processes even outside the R&D or GMP areas.
R&D needs flexibility and should not be over-regulated, whereas GMP concentrates on standardization and high throughput. On the other hand, R&D has to create a solid and robust fundament to enable manufacturing and quality control to work according to established and efficient procedures.
The training aims to generate a common understanding of how to best interlink these two areas and create highly efficient processes from product development to routine manufacturing of
generic pharmaceutical products.
Development of generics: From R&D to GMP In-house Training
EVENT INTRODUCTION
The interface between Development and GMP units is one of the most exciting in the pharmaceutical industry. Mutual understanding of the different regulatory environment and the functional priorities is a key success factor. Conflicts, mostly resulting from different approaches and priorities, can have negative impact on business processes even outside the R&D or GMP areas.
R&D needs flexibility and should not be over-regulated, whereas GMP concentrates on standardization and high throughput. On the other hand, R&D has to create a solid and robust fundament to enable manufacturing and quality control to work according to established and efficient procedures.
The training aims to generate a common understanding of how to best interlink these two areas and create highly efficient processes from product development to routine manufacturing of
generic pharmaceutical products.
Who should attend?
• Heads of R&D
• Manufacturing Heads
• Heads of QC
• Heads of QA
• Pharmaceutical Development managers
• Manufacturing managers
• Stability control managers
• QC managers
• Drug Regulatory Affairs managers
• Product Maintenance managers
REQUEST A TAILOR-MADE OFFER
Past Clients
Testimonials
„
The level of professionalism of the trainer. Fine-tuned program without wasting time
„
Managing Director – European pharma company
Testimonials
„
Clear and interesting way of presenting issues, good examples and very knowledgeable trainer.
„
HR Director - Multinational company based in Europe
Testimonials
„
Excellent trainer, super skilled, funny and extremely dynamic
„

M&A Professional
Testimonials
„
This training was interesting and I would love to attend more trainings like this.
„

HR Professional
Testimonials
„
All lectures of the trainer was exactly what I was looking for and it was directly related to my experties and area that I am working. On top of that he prepared all lectures in a very practical way and also took his time to asnwers to the questions and he made sure that we got the asnwer. The organization of this class was also very good.
„

Pharmaceutical professional
Testimonials
„
Comprehensive overview with very knowledgeable and experienced trainers. The break-out sessions were great as they made the course more interactive and allowed us to apply the concepts in practice.
„

Banking professional from Europe
About GLC EUROPE
Global Leading Conferences is specialized in designing, creating and executing impactful, and pragmatic Conferences, Masterclasses and in-house training.
We take pride in empowering thousands of professionals around the world to learn, network, make connections, and do business with thought leaders and visionaries across multiple industries.
GLC actively collaborates with professionals in major sectors, such as Finance, Pharmaceutical, Human Resources, Health & Safety and Energy to understand the challenges and issues that each industry endures, to be able to construct exclusive and top-tier learning and networking platforms.
Specialized in planning, designing, and organizing interactive, impactful, and pragmatic B2B events, GLC actively collaborates with professionals in major sectors, such as Finance, Pharmaceutical, Human Resources, Health & Safety, and Energy to understand the challenges and issues that each industry endures.
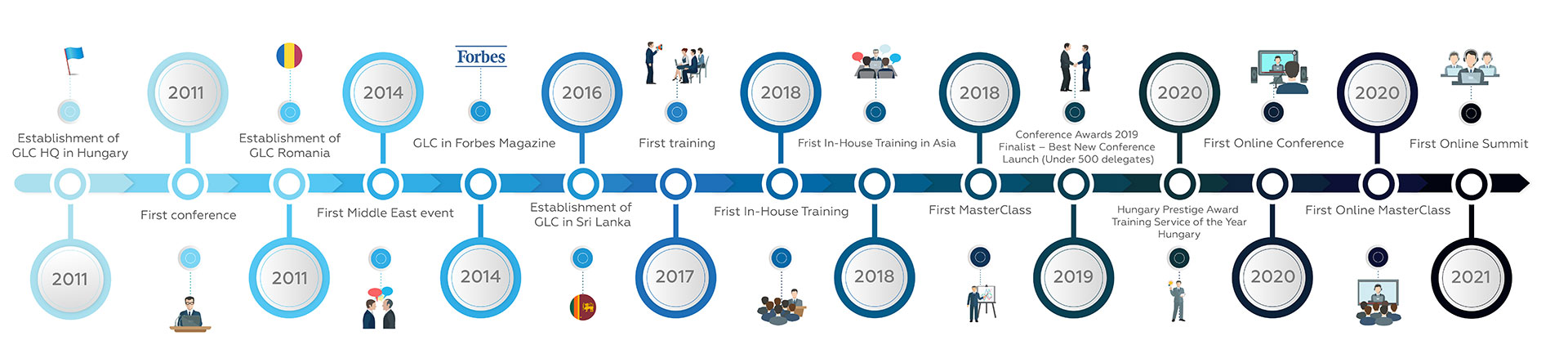
10
years
of constructing industry focused events where business meets intelligence
299
events
where we have been learning to provide a unique experience for our customers
3410
speakers
from top-notch industry establishments proven to make a difference
17761
clients
thereof 96% would attend a GLC event again and would recommend GLC
The MANAGEMENT

GHAZAL ZOHREHNEJAD
Managing Director / Co-founder

LÁSZLÓ ÁRVAI
Managing Director / Co-founder

KRISZTIÁN ÖTVÖS
Managing Director / Co-founder













