Safety Data Exchange Agreements (SDEAs) and PV Arrangements with External Vendors MasterClass
Online MasterClass | 17 & 18 November, 2021
It is common in our globalized world that, according to their specific and particular strengths and interests more than one pharmaceutical is involved in the marketing of a pharmaceutical product, even in the same geographical area or country. It is crucial and tightly required by the Regulatory Authorities that the safety information arising at any point is adequately captured, distributed and adequately and timely reported. The risk benefit ratio should be maintained at all times and for this reason agreements between pharmaceutical companies marketing a certain product should include agreements (so called Safety Data Exchange Agreements, SDEA) to ensure the adequate flow of any arising safety concerns. This should also be considered in all outsourced activities of a pharmaceutical company that may lead to any capture of safety information related to the products of which the company is the Marketing Authorization Holder (MAH)
Safety Data Exchange Agreements (SDEAs) and PV Arrangements with External Vendors MasterClass
Online MasterClass | 17 & 18 November, 2021
EVENT INTRODUCTION
It is common in our globalized world that, according to their specific and particular strengths and interests more than one pharmaceutical is involved in the marketing of a pharmaceutical product, even in the same geographical area or country. It is crucial and tightly required by the Regulatory Authorities that the safety information arising at any point is adequately captured, distributed and adequately and timely reported. The risk benefit ratio should be maintained at all times and for this reason agreements between pharmaceutical companies marketing a certain product should include agreements (so called Safety Data Exchange Agreements, SDEA) to ensure the adequate flow of any arising safety concerns. This should also be considered in all outsourced activities of a pharmaceutical company that may lead to any capture of safety information related to the products of which the company is the Marketing Authorization Holder (MAH)
Who Should Attend?
• Pharmacovigilance professionals • Auditors and PV Auditors • Medical Affairs Professionals involved in outsourced Medical Activities as Advisory Boards, Congresses, etc • Marketing Professionals working in outsourcing activities • Legal Department Professionals
Past Clients
Testimonials
„
The level of professionalism of the trainer. Fine-tuned program without wasting time
„
Managing Director – European pharma company
Testimonials
„
Clear and interesting way of presenting issues, good examples and very knowledgeable trainer.
„
HR Director - Multinational company based in Europe
Testimonials
„
Excellent trainer, super skilled, funny and extremely dynamic
„

M&A Professional
Testimonials
„
This training was interesting and I would love to attend more trainings like this.
„

HR Professional
Testimonials
„
All lectures of the trainer was exactly what I was looking for and it was directly related to my experties and area that I am working. On top of that he prepared all lectures in a very practical way and also took his time to asnwers to the questions and he made sure that we got the asnwer. The organization of this class was also very good.
„

Pharmaceutical professional
Testimonials
„
Comprehensive overview with very knowledgeable and experienced trainers. The break-out sessions were great as they made the course more interactive and allowed us to apply the concepts in practice.
„

Banking professional from Europe
About GLC EUROPE
Global Leading Conferences is specialized in designing, creating and executing impactful, and pragmatic Conferences, Masterclasses and in-house training.
We take pride in empowering thousands of professionals around the world to learn, network, make connections, and do business with thought leaders and visionaries across multiple industries.
GLC actively collaborates with professionals in major sectors, such as Finance, Pharmaceutical, Human Resources, Health & Safety and Energy to understand the challenges and issues that each industry endures, to be able to construct exclusive and top-tier learning and networking platforms.
Specialized in planning, designing, and organizing interactive, impactful, and pragmatic B2B events, GLC actively collaborates with professionals in major sectors, such as Finance, Pharmaceutical, Human Resources, Health & Safety, and Energy to understand the challenges and issues that each industry endures.
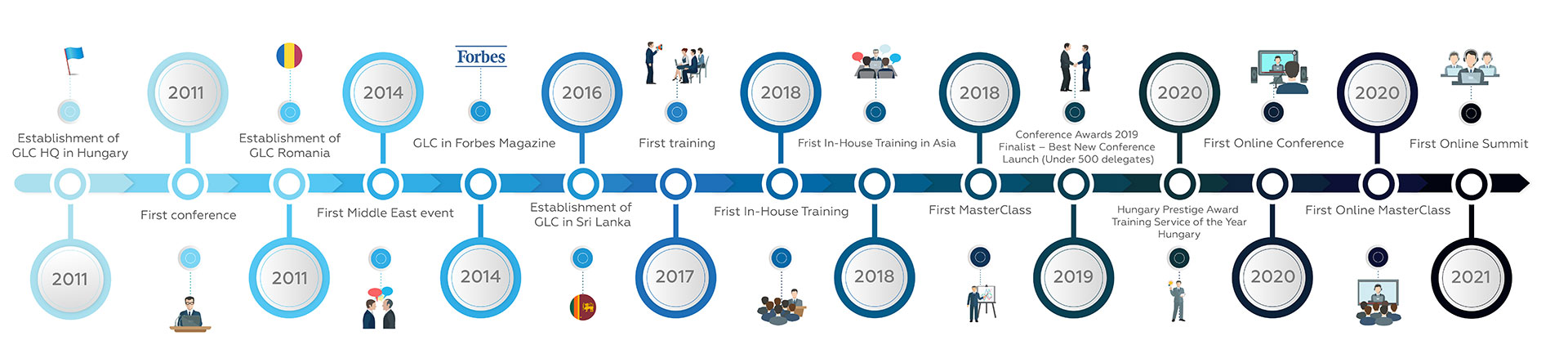
10
years
of constructing industry focused events where business meets intelligence
299
events
where we have been learning to provide a unique experience for our customers
3410
speakers
from top-notch industry establishments proven to make a difference
17761
clients
thereof 96% would attend a GLC event again and would recommend GLC
The MANAGEMENT

GHAZAL ZOHREHNEJAD
Managing Director / Co-founder

LÁSZLÓ ÁRVAI
Managing Director / Co-founder

KRISZTIÁN ÖTVÖS
Managing Director / Co-founder













